Engineering Principles for Maintenance cont.
- 1. Engineering Principles for Maintainers and Operators.
- 1.1. Work within the design limits
- 1.2. Force transmission
- 1.3. Stress in materials
- 1.4. Clearance, fits and tolerances
- 1.5. Bending stress
- 1.6. Torque stress and combined loads
- 1.7. Metal fatigue failure
- 1.8. Lubrication
- 1.9. Vibration
- 1.10. Chemical and material compatibility
- 1.11. Corrosion
- 1.12. Fluid flow
- 1.13. Cavitation
- 1.14. Welding metals
- 1.15. Welding plastics
- 1.16. Heat transfer
- 1.17. Bearing isolation and protection
- 1.18. Electric motors
- 1.19. Process control and monitoring
- 2. Equipment Maintenance Best Practice.
- 2.1 Creative disassembly
- 2.2 Bolting and gaskets in flanged connections
- 2.3 Threaded connections
- 2.4 Chain and sprocket drives
- 2.5 V-belt and pulley drives
- 2.6 Mechanical seals
- 2.7 Soft-foot distortion
- 2.8 Shaft alignment
- 2.9 Pneumatic valve actuators
- 2.10 Process control valves
- 2.11 Isolation valves
- 2.12 Roller bearings
- 2.13 Oil cleanliness
- 2.14 O-ring seals
- 2.15 Gear box drives
- 2.16 Gland packing
- 2.17 Vibration control
- 2.18 Non-destructive testing of welds
- 3. Types of Maintenance Philosophies.
- 4. Maintenance Management and Asset Management.
- 5. Fault Finding and Troubleshooting Check Lists

10 More Principles of Applied Engineering for Maintenance Personnel.
Excerpt from the Industrial Maintenance Technician's Guide.
Below is a contention of chapter 1 (Engineering Principles for Maintainers and Operators) of the free online industrial maintenance technician guide. Applied engineering principles explained on this page are Vibration, Chemical and Material Compatibility, Corrosion, Fluid Flow, Cavitations, Welding Metals, Welding Plastics, Heat Transfer, Bearing Protection, Electric Motors, and Process Control.
If you would like to reference this guide offline, and/or print copies for your maintenance, operators, or students in your facility, you can purchase a PDF version of this guide at the bottom of this page.
1. Engineering Principles for Maintainers and Operators (cont.).
1.9 Vibration
Vibration in equipment is the result of unbalanced forces. The transference of unbalanced forces through equipment into neighboring structures causing them to shake is vibration. The motion of a body is limited by what connects it to a machine and the walls in which it moves. Every time there is a change of direction unbalanced forces produce a shock. This shock travels throughout the machine and is transmitted to all connected items. Out-of-balance is corrected by adding or removing material so that when the equipment is operating the unbalance is controlled to an acceptable level.
The rate of vibration is called the frequency. It is measured in cycles per second and has the units Hertz. A four-pole electric motor rotates at about 1500 RPM. This is 25 cycles per second or 25 Hertz. Vibration caused by an external applied force is known as a forced vibration because the mass oscillates at the frequency of the external force. An example is the shake produced by the moving pistons and crankshaft in a car engine.
The four methods of vibration control are listed below.
- Reduce or eliminate the exciting force by balance or removal.
- Use sufficient damping to limit amplitude.
- Isolate the vibration source from the surrounds by using spring mounts of appropriate stiffness.
- Introduce a counterbalancing force opposite in phase to the exciting force.

Most important engineering principle applied to this situation is that every moving mass must be balanced about its center of rotation. Every rotating mass must be balanced to an acceptable standard. Out-of-balance rotors cause vibration because the center of mass of the rotor is eccentric (not running true) to its center of rotation. The spinning, off-center mass is continually being flung outward. The machine's bearings hold the mass in place and react against the developed forces. Vibration results as first the mass is on one side of the bearings and then it is on the other. Balancing aims to distribute the mass evenly about the running center. The drawing above shows eccentricity between the center of mass and the center of rotation.
Materials such as rubber dampen shaking. The rubber flexes and absorbs the movement within itself. Rubber makes a good vibration damper. Because rubber cannot compress much to accommodate movement, rubber dampers are normally used for low amplitude, high frequency vibration where noise transference is a problem. Shock absorbers are used for large amplitude, low frequency situations where springs alone would produce bouncing. An example is in car suspensions.
A vibrating mass can also be isolated from its surroundings by springs. The springs deflect under the shaking body. Installing isolation springs make the spring's natural frequency the governing frequency for forced vibration transfer. How far a spring will compress under load depends on its stiffness. Altering the spring stiffness controls the amount of vibration transferred to the attachment. Taking into consideration another applied engineering principle, that too stiff will transmit vibration, while insufficient stiffness will cause bounce. The correct spring stiffness can be found using charts available from specialist vibration control companies.
An out-of-phase mass is a method not often used to control vibration. Anther applied engineering principle is that it is possible to use a weight with an opposite vibration pattern to negate the out-of-balance forces. This method has been used in motor car engines where a shaft with an eccentric mass is spun in the opposite direction to the crankshaft.
Causes of out-of-balance
The table below lists some common causes for unbalance.
a) Bent or bowed between support bearings
b) Overhung weight bends shaft under gravity
c) Unevenly distributed solid or liquid inside rotor
d) Loose parts on the rotor
e) Eccentrically manufactured diameters on the rotor
f)Misalignment of the drive train to the rotor axis
g) Loose drive couplings flop about
h) Loose tolerances between assembled parts on rotor
i)Shoulders on rotor are made out-of-square to axis
j)Voids or cavities within the rotor
k) Misalignment of bearings force shaft to bow
The drawings below provide examples of some of the problems listed in the table above.
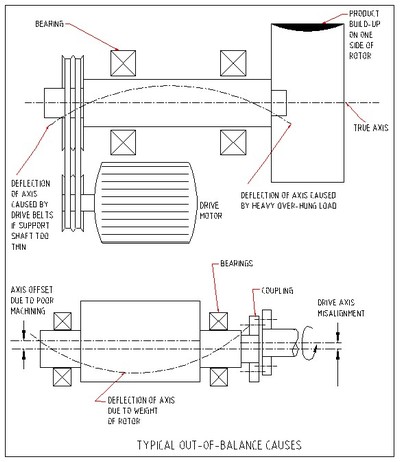
Minimizing vibration involves minimizing out-of-balance forces. The following table indicates simple actions to take to reduce the problems of out-of-balance.
i)Make the rotor with all diameters on the same axis
ii) Machine the rotor from one piece of material
iii) Machine the rotor complete without altering the initial machining set-up
iv) Finish machine multiple part rotors when fully assembled on the shaft
v) Reduce lengths of unsupported sections and overhangs
vi) Keep tolerances tight on parts assembled on the rotor
vii)Align the drive train to the axis of the rotor carefully
viii) Insure the bearing supports are aligned
Balancing rotors
An out-of -balance machine can be made to run smoothly by balancing the rotating parts to within acceptable limits. Balancing is the process of attempting to improve the distribution of mass in a body so that it rotates in its bearings without unbalanced centrifugal forces. It can only be attained to a certain degree. Attaching or removing weight at relevant points on the rotor permits balance corrections. For example specifically weighted tags can be welded to the rotor, or metal can be removed by drilling holes into the rotor at relevant positions.
When a part is to be balanced it is necessary to specify the quality of balance required. The more precise the balance required the higher the cost to attain it. Relevant standards have been set for various types of components.
To minimize the possibility of vibration problems, specify to the machine shop the degree of balance quality required of the part. To improve the accuracy of balancing send all the attached assemblies such as couplings, bolts, keys and pulleys with the rotor so the effect of the components can be allowed for in the balance corrections.
1.10 Chemical and Material Compatibility
Chemicals can attack and destroy the materials in contact with them. Chemical attack is the result of the reaction of process chemicals on the materials confining them. When corrosive and hazardous chemicals escape their confinement and get into the work place and environment they become dangerous, life threatening dangers. Pipes and vessels can be eaten through, gaskets and seals can be degraded and leak, equipment parts can be attacked and fail. Even water will attack and destroy materials if they are wrongly chosen. Chemical compatibility, which is selecting the right materials to contain a chemical, is the first critically important step in managing and handling chemicals safely.
Chemical corrosion is the name usually used to describe chemical attack of metals. As the name implies the chemicals chemically react with the metal and remove metal from the confinement. Chemical degradation is used to describe chemical attack on non-metals. The chemical can attack the non-metal or the bonding agent holding the material together.
Over the years, through laboratory experimentation and field trials, chemical compatibility tables have been put together for the chemicals used in industry. In the case of a new chemical, the chemist can usually deduce its properties from its chemical make-up or check them in small-scale laboratory trials. Compatibility tables are available from the chemical supplier and from the equipment supplier.
There are three common options available when using materials in contact with chemicals.
§ The entire equipment item can be made of a chemically resistant material that has indefinite life.
§ The equipment item can be coated in a chemically resistant coating separating the chemical from the structure.
§ The equipment can be made of non-resistant material but the corrosion is kept at a known and manageable rate and the item is replaced or repaired before the end of its working life.
Select resistant materials
Using more resistant materials will reduce the effect of chemical attack. Specialist metals and non-metals to contain aggressive environments are available. These can be expensive and their use is based on their cost effectiveness. Often an additional 20% cost provides a corrosion life 5 times longer. A difficulty when selecting specialist alloys for agitated conditions is the availability of the metal in the form required, at an acceptable price. Sometimes compromises are necessary between availability, price and longevity.
Compatible coatings and linings
Coating in a material unaffected by the chemical can prevent chemical attack. Rubber lining of hydrochloric acid storage tanks and plastic lining of process piping are examples. Teflon coating can protect bolts and nuts. Rubber linings normally fail at joins. Check the correct procedure is used when they are mounted in place.
Always test the coating to prove there are no holes. High voltage spark testing is used on thick coatings and linings while the low voltage 'wet sponge'method is used on thin coatings.
Acceptable corrosion
At times chemical corrosion is acceptable and one need only allow for it by using thicker materials or slowly degrading materials. An example is the storage of sulphuric acid in mild steel tanks at ambient conditions and concentrations higher than 80%. Though the acid attacks the metal, the rate of corrosion is extremely slow. Using thick walled steel tanks with a corrosion allowance which takes decades to thin can be a cost-effective option.
Changed material properties
Heat from welding processes alters the metal properties at the heat-affected zone (HAZ) of the weld. Stresses are introduced into the metal and grain microstructures are altered. Consideration of the welding method, weld procedure and use of stress relief can mitigate the effects. The effect of welding is particularly evident when hot caustic solutions are contained in mild steel vessels. Unless stress relieved, chemical attack can occur at the HAZ of the welds.
Consider process conditions
Using corrosion tables without considering all the process conditions can lead to poor materials selection. Most published data on compatibility do not take into affect agitated conditions. Chemical attack occurs at increased rates in agitated conditions. Agitated conditions include stirred vessels and tanks, mixers and agitators, pumps, pipelines and valves. It is necessary to allow for the affect of agitation when selecting materials of construction. When selecting valves for agitated conditions chose chemically compatible materials with wear resistance, which minimize turbulence and meet the pressure requirements of the process. Some data only applies to room temperatures. Data is normally not available on the effect of aeration. Nor is data readily available on the effect of other contaminants, for example chlorides with stainless steels.
Alternate materials
In looking at better material selection for corrosive services it is useful to consider the use of non-metals. Plastics can have excellent corrosion properties. Provided their temperature limits are respected they can be used to line the contact surfaces. Plastic lined pipes in corrosive service are common. Corrosion resistant bricks and mortars have been successfully used as linings. Fiberglass can be used provided the corrosion barrier layer is not worn away by abrasion. Rubber lined valves, such as diaphragm valves, have been used for agitated corrosive conditions for decades. Rubber lined agitators have been installed into reactors. The internals of pumps and pipes have been successfully protected with specialist epoxy coatings.
In uncertain or if changeable situations will occur in a vessel, consult the material manufacturer and ask for their advice. If they cannot help, then the only remaining option is to conduct your own laboratory tests or field trials.
Chemical corrosion control and mitigation requires creative use of a few basic principles.
1.11 Metal Corrosion
Metal corrosion is a chemical reaction between a metal surface and its environment. Corrosion can occur in a gaseous (dry) environment or a damp (wet) environment. It is the result of an electrical process in which electrons are exchanged between the corroding metal atoms and the acceptor element atoms. Corrosion control involves stopping or minimizing the loss of electrons between the components involved in the process.
Corrosion in a gaseous environment produces a surface layer of converted metal. The oxide layer shields the metal from the oxygen and forms a barrier. The oxide will not react with the oxygen in the air or the metal. Provided the oxide layer does not crack, or is not removed, the metal is protected from further corrosion. But if the bare metal is exposed to the oxygen, it will again react to form the oxide. In this case the presence of oxygen benefits the metal's protection. Removal of the oxygen removes the metal's ability to create its own protective corrosion barrier.
Corrosion in a wet environment attacks the metal by removing the atoms on the metal surface. In the wet corrosion process the electrons from the corroding metal (anode) move to the connected cathode where they recombine to make a stable compound with the positively charged metal ions (M++) that originally lost the electrons. The moisture provides the electrons with a continuous pathway to escape the parent metal. And the parent metal, which cannot develop a protective barrier, falls apart. Once corrosion starts it continues until the ingredients in the corroding metal are all used up.
THE ELECTRICAL NATURE OF CORROSION
A flow of electrons means there is an electric current. Wet corrosion produces a corrosion cell. Much like a car battery. The electrons used in creating the corrosion product are continually replaced from the corroding metal and the atom in the metal fall out one by one. The numbers of electrons available for reacting control the amount of current developed between the two metals. The anode cannot corrode unless there is a cathode. One of them will control the rate of electron flow and thus the corrosion rate.
The intensity (the number of electron and positive ion pairs) is dependent on the potential difference, or voltage, which exists between the metals and the surface area of each metal. Different metal combinations have different voltage potentials between them. Joining two metals with a large potential difference between them produces higher corrosion rates than if the metals were close in electrical potential. Corrosion of dissimilar metals is known as galvanic corrosion.
Surface area effects
The size of the cathode relative to the anode is important. A large cathode has more surface area through which electrons can flow and so develops an intense electric current with the anode (corroding metal). A small anode connected to it is forced to supply these electrons and will quickly corrode and fall apart. Whereas a large anode connected to a small cathode can provide electrons from any location and will take a long time to show evidence of corrosion.
Where a less noble (base, anodic) metal has to be in contact with a noble metal make sure the less noble metal has at least one hundred times more surface area than the noble metal. Remember 'large anode, small cathode 'not the opposite.
Differential aeration effects
The corrosion reaction requires oxygen, where oxygen is present the metal is cathodic, and where oxygen is depleted the metal is anodic and corrodes. The parts of the metal in contact with the highest oxygen concentration become cathodic and are protected, and the areas where oxygen concentration is low will corrode. Steel posts dug into the ground will rust just below the surface because of this effect.
Stagnation effects
During corrosion, ions build up immediately around the anode and cathode saturating their respective regions. The corrosion rate begins to fall due to the concentration of stagnant ions blocking the creation of more ions in the electrolyte. If the ions are removed or more voltage is provided the corrosion rate again picks up. If you want fast corrosion then agitate the electrolyte and add oxygen.
CONTROLLING CORROSION
Corrosion control involves hindering the natural chemical reactions that occur between the metal and its environment.
Modify the environment
Removing oxygen from the environment prevents completion of the corrosion process by slowing the chemical reaction requiring electrons. If oxygen can be kept away from the protected cathode then the electrons cannot readily flow, so causing the current to drop and corrosion to slow.
Another technique is to use corrosion inhibitors that combine with the corroding metal (anode) or the protected metal (cathode) to form a barrier layer that reduces the flow of ions and electrons across it to very low values and virtually stops the corrosion. If the protective barrier layer is damaged corrosion restarts, so it is necessary to keep an amount of the inhibitor in contact with the metals. This is a common technique used in boilers to protect them from corrosion.
Modify the properties of the metal
From the galvanic series we can see that the more noble metals are less likely to corrode. When these metals are metallurgically combined with those from lower in the series, the resulting alloy takes on corrosion resistant properties. The resistance can come from the development of a protective oxide film on the outside surface or because the new alloy has a different voltage potential which acts to make it behave more noble.
Passivation of a metal is a method of changing the potential difference of a metal's surface. By removing the oxide layer normally present on a metal and exposing the bare metal directly with an acid, the acid reacts with the metal surface to make a new compound with more noble electrical properties. The passivity layer covers the metal, and provided the layer is not broken and the voltage potential remains favorable, it will protect the metal under it from corrosion.
Put protective coating over the metal
Metals can be protected by covering them in a coating of a different material with better corrosion resistant properties. Metallic and non-metallic coatings are used.
Metallic coatings of less noble metal over more noble metal provide sacrificial protection. Galvanizing is a bonded, protective coat of zinc put over steel. The zinc protects the steel from corrosion in two ways. From the galvanic series it can be seen that zinc will corrode before steel (sacrificial). Secondly, a protective layer of zinc oxide forms on the zinc. If the oxide layer is scratched the zinc is exposed to oxygen and the oxide layer reforms. If part of the zinc coat is lost the rest of the bonded zinc starts to corrode in preference to the steel. As long as the zinc remains in contact with the steel it corrodes sacrificially and protects the steel.
Non-metallic coatings put over a metal can be of two types. They can act as a physical barrier and bar access to the metal surface or they can introduce a very high resistance into the corrosion cell circuit and drastically reduce the flow of electrons. The barrier type coatings protect the metal as long as there are no cracks. If a crack occurs corrosion becomes intense at the metal surface. Resistance type coatings include additives that breakdown in the presence of water and oxygen into inhibiting agents.
Impose an electric current
The following applied engineering principle is often overlooked by the novice, so please take note. Wet corrosion produces a bi-metallic cell and an electrical current of moving electrons flow from the less noble, anodic metal. If instead the electrons were supplied from another source the less noble metal would not corrode first. This technique is known as cathodic protection. By connecting a more anodic metal into the corrosion circuit than the metal to be protected, the more anodic metal will corrode first and provide an alternate source of electrons. This is why zinc blocks are placed on ship hulls to protect any steel in contact with seawater. Alternatively a metered electrical current from a power source can be connected to the cathode to supply the electrons.
Change to non-metallic materials
There are many other materials that can be used instead of metals in situations where metallic corrosion is expected. Provided the physical properties of the non-metal are satisfactory their use may prove a more effective choice.
PRACTICES TO ADOPT TO REDUCE CORROSION
ü Clean out debris from the bottom of metal sumps exposed to the atmosphere to prevent oxygen depletion under the debris.
ü Repair any damage to painted surfaces quickly to seal the metal off from atmospheric oxygen and moisture.
ü Don't create galvanic cells by mixing metals. Check the galvanic series when ordering equipment and parts to see if components are compatible. Keep them dry and out of the weather.
ü Check the right type of paint is being used with good corrosion protective properties.
ü Grout under base plates completely and finish the grout at the edges of the lower face. Do not bring it up the side of the base plate and create a crevice.
ü Remove a paint blister immediately, clean the metal thoroughly and repaint to prevent differential aeration.
ü Before bolting or clamping metals parts together insure they both have a completely protected surface. This can be with a sacrificial coating or a barrier coating.
ü Be aware that electric currents can flow for hundreds of meters if parts are joined by an electrolyte. Underground pipes in moist soils need to be protected from bother oxygen depletion and galvanic action from dissimilar metals anywhere along their route.
ü Use a generous radius when bending metals to minimize the creation of internal tensile stresses.
ü Use deionized water when washing down components made of dissimilar metals in contact and dry them off quickly and thoroughly. This is especially important with coils made of dissimilar metals.
ü Protect underground piping from direct contact with soil and protect the coating from damage. The smallest penetration to the metal will result in rapid pitting corrosion of the pipe wall. If that is not possible install cathodic protection. Beware that some painted coatings still allow moisture and oxygen through to the metal. Check the corrosion protection properties of the coating.
1.12 Fluid flow
A fluid is either a liquid or a gas. This engineering principle is applied in industry when they are piped from storage to the point of use. Correct design and installation of the piping system minimizes pressure loss and improves the behavior of equipment and processes.
The pipe wall
A fluid flowing through a pipe contacts the pipe wall. The pipe wall has surface roughness. The amount of roughness affects the drag on the fluid. In the valleys between projections the fluid moves slowly. Above the projections it moves faster. The drag between layers tears, or shears, them apart and each layer moves at a different speed. The velocity at the wall is zero and fastest at the center.
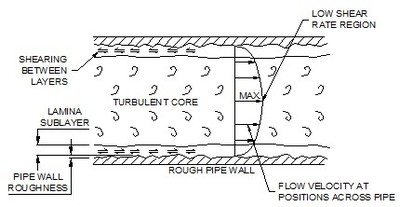
Friction and the laminar sub layer
Because of friction caused by the pipe wall the fluid moves slower near the wall. This slow moving fluid is known as the laminar sub-layer. The sub-layer only develops in turbulent (fast) flows. At slow flows the sub-layer blends in with the lamina (slow) flow in the pipe. Figure 1 shows the effect on flow velocity of the surface of a pipe wall.
Away from the pipe wall the flow is turbulent. In this area there are eddies and vortices moving randomly about the pipe from side to side and top to bottom. It is a region where confused lumps of fluid 'rattle'about their way along the pipe. Between the laminar and turbulent regions is a short transition zone as the flow changes to turbulent.
Viscosity and density effects
Liquids do not all behave the same. Blood has different flow characteristics than water. Paint flows differently to gasoline petrol. Liquids are categorized by their behaviors when undergoing shear. The shear rate is a measure of a fluid's viscosity or slipperiness.
The density of a fluid affects its viscosity. Fluids with more mass per unit volume are heavier and require more energy to move them and shear less easily. A temperature rise decreases the viscosity and density of liquids. The more viscous, or less slippery, a fluid the harder it is to get shearing between layers. The high viscosity prevents rapid velocity changes occurring between layers and makes it harder to pump.
Velocity effects
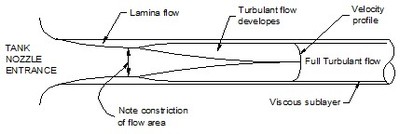
At low speeds the whole flow across a pipe is laminar and the fluid slides over itself. As the speed becomes faster eddies start to form and cross the fluid layers. A transition from laminar to turbulent flow develops. At still higher velocities the flow in the core of the pipe becomes turbulent with swirling eddies throughout. Figure 2 shows where the various flow regions occur at a tank nozzle.
The laminar sub layer is always present against the pipe wall. But as the velocity rises the energetic swirling eddies begin to impact more deeply and the sub layer begins to thin. At still higher velocities the sub layer thins further and the taller roughness peaks stick into the turbulent region. Where the sub layer covers the roughness projections the wall is considered 'smooth'. When the wall roughness pokes out of the sub layer the wall is considered 'rough'. This means the same wall can be both smooth and rough depending on the fluid's velocity.
Experiments have proven the pressure loss along a pipe with laminar flow is proportional to the velocity (p µ V) where as for turbulent flow the pressure loss is greater and proportional to the square of the velocity (p µ V2). A slower flow permits a thicker sub layer and creates a 'smooth'pipe wall. This minimizes the losses along the pipe. There is a very much greater loss of pressure in turbulent flow.
Minor losses in pipe fittings
Elbows, bends, reducers, branch tees and flanges all cause individual minor pressure losses. When a fluid is forced to change direction, or go around a disruption, eddies are produced. These new twisting eddies interfere with the flow pattern and produce additional pressure losses. The greatest pressure losses occur at sudden diameter and direction changes. Most of the loss occurs in the downstream eddy wake. When designing a pipe run gradually blend-in changes to the flow pattern.
Gas flow
Unlike a liquid a gas is compressible and can be squashed. When a gas is compressed the density increases. As the pressure is released the density decreases. Gas flowing into a pipe starts at a pressure, temperature and associated density. The frictional losses along the pipe cause a pressure loss. If the gas is now at a lower pressure it must be at a correspondingly lesser density. (It is less squashed together than it was at the start.) This means the density of a flowing gas varies along the length of the pipe. The effect is greater at higher velocities.
For a mass of gas to enter a pipe an equal mass must leave the pipe. We know the density is continually thinning as the pressure drops along the pipe. One kilogram of less dense gas requires more space (volume) than the same weight of a more compressed gas. To get one kilogram of expanding gas, which is taking up more volume, out from the end of the pipe it must go faster than when it entered the pipe. Gas flowing through a pipe expands as the pressure falls and speeds up the further it travels along the pipe.
Expanding gas cools. This engineering principle is applied in refrigerators and air conditioners. A gas flowing in a pipe is expanding as the density falls. This is why compressed air lines are cool to touch and why water droplets collect in pneumatic valve actuators. The temperature has fallen low enough to condense the water vapor.
WHERE PRESSURE LOSSES COME FROM IN PIPES
Liquid moving in a pipe has to push its way along pipe walls, around bends, through valves, past projections and enclosed items. Throughout its progress friction robs energy. Three factors affect pressure loss in pipes.
Surface Friction
Along walls the liquid has to overcome friction. Wall friction depends on surface roughness. The higher the projections into the liquid the more the friction. At low velocities a laminar sub layer of slow flowing liquid covers the roughness. But at high velocities the sub layer thins as eddies in the turbulent center flow extend to the projections.
Direction Changes
Liquid that is forced to change direction loses energy. When a liquid goes around a 90o bend its momentum has to be redirected. From going at velocity in one direction it now has to go at the same velocity in a completely different direction. To do this it converts pressure into the energy it needs to change direction. A pressure loss results. The more sudden the change in direction the greater the energy it needs and the higher the corresponding pressure losses.
Liquid viscosity
A liquid that easily slides requires less energy to move than one that does not want to flow. Honey is thousands of times more viscous than water. Pushing honey through pipes, around corners and past valves requires far more energy than for water. If the honey was warmed it would then flow easier and the pressure loss would be less.
MINIMISE FRICTION IN PIPELINE CONSTRUCTION
During design, procurement and installation of a pipeline follow the recommendations in Table 1.
DESIGN
Select smooth bore pipes.
100 meters of 100 mm (4') plastic pipe containing water moving at 1 m/s (3.25 ft/s) will lose 900 mm (35') of pressure. The same flow in a 100 mm steel seamless pipe will lose 1000 mm (39') pressure.
Minimize flow disruptions.
Use long radius elbows; use pipe reducers; minimize the number of flanges; use full bore straight through valves; use pipe size branch tees and reduce down on the branch (a must on suction lines); use instrumentation which does not project into the flow.
Keep flow velocities low.
Select pipe sizes that are practicable while keeping flow velocities reasonable.
CONSTRUCTION
Cut gasket holes to the bore diameter.
Make the gasket bore hole to the pipe bore diameter. Do not let the gasket project into the liquid flow path, as it will cause unnecessary friction and turbulence.
Clean off weld splatter.
Weld splatter sits on the pipe surface and disrupts the liquid flow pattern. The splatter can come lose and damage down stream instruments and equipment.
Keep butt weld peaks low.
Like gaskets cut with the diameter less than the pipe bore, welds with high peaks project into the liquid flow. Try keep welds below the height of the sub-layer thickness.
Make multiple segment mitered bends.
'Lobster back'90o bends are used when large diameter long radius bends are not available. Make them out of equal sized 15o segments instead of the usual 22.5o segments
TABLE 1 Practices that minimize pressure loss in pipes.
1.13 Cavitation
What is cavitation?
Cavitation is the occurrence of vapor bubbles in a liquid. A vapor bubble will form when the pressure in a liquid falls so low it boils or the temperature rises so high it boils.
Applied Engineering Principle:
Vapor bubbles can only occur if pressures are sufficiently low or temperatures sufficiently high. Until these conditions are reached there is only liquid and when exceeded there is only vapor. Water will boil at 100 oC (212 oF) at sea level pressure of one atmosphere or 101kPa (14.7 psi). Drop the pressure to 50% of an atmosphere by creating a partial vacuum and it will boil at 82 oC (180 oF). Drop it to 2% of an atmosphere (98% vacuum) and the water boils at 18 oC (64 oF). Each liquid boils at temperatures distinct to themselves.
If you look at a saucepan of boiling water on the stove you will see vapor bubbles rising from the bottom of the pan and exploding at the surface. They start small at nucleation sites on the saucepan floor and grow bigger as the pressure of liquid overhead decreases during their rise. The saucepan is cavitating right in the kitchen. We call it boiling. The same situation develops in a cavitating pump. Cavitation can occur anywhere that the right conditions develop. Examples are downstream of control valves as the liquid ejects out, the tips of propellers and agitators moving at high speed, downstream side of instrument probes in pipes containing fast moving liquid and in pump impellers.
Cavitation becomes a problem when the bubbles cause damage to internal parts from implosions as the pressure again increases or when the vapor bubbles block off the available liquid flow area and reduce capacity.
What causes the pressure to drop so low?
Bernoulli's Law states that the higher the speed of a moving liquid, or gas, the lower the pressure. A pump moves large volumes of liquid quickly from the suction inlet to the discharge outlet. If the liquid's speed through the pump becomes fast enough to create sufficiently low-pressure conditions it will boil off the liquid and form vapour bubbles.
But the trouble starts even before we get to the pump. Each inch and millimeter of suction pipe, each elbow, every valve, each strainer, all protrusions and surface roughness, any change of flow direction before the pump - rob it of pressure. Friction lose robs the liquid of energy. Even worse is when the pump has to suck liquid from a location at lower pressure or at a lower level than itself. For the liquid to move to the pump, the pump has to make a vacuum. The pressure in the pump has to fall low enough to suck the liquid from the low-pressure region. This greatly reduces the pressure range left before the liquid starts to boil.
How is cavitation damage caused?
The vapour bubbles collapse when the pressure in the liquid increases. The implosion occurs in milliseconds and microscopic 'torpedoes'of liquid are ejected at up to 500 m/s (1650 ft/sec). Continuous impact on pump internals gradually shred the metal surface. The erosion process is accelerated in corrosive situations as the jet impact removes the corrosion coating and exposes fresh metal to the liquid. The whip cracking sounds heard when cavitation occurs is a result of the impact from the opposing walls of water in collapsing bubbles. An impeller suffering severe cavitation is full of ragged edged holes and looks like worms have attacked it. Early signs of cavitation show up as pitted and roughened patches in localized areas just down stream of the low-pressure areas.
WHAT CAN BE DONE TO REDUCE CAVITATION?
To stop or reduce cavitation we need to maintain pressure above vapor pressure. This can be achieved by pressurizing the suction line, by cooling the liquid and by reducing the friction losses. Because the velocity directly affects pressure the first thing that can be done is to close off a valve on the discharge of the pump and slow the liquid down. Another way to raise the pressure on the suction side is to raise the liquid level at the source.
Often cavitation is created at the engineer's drawing board from overlooking key applied engineering principles. Small bore pipes and oversized impellers cause greater flow velocities than necessary. The high velocity will cause high friction, which produces high-pressure loss resulting in cavitation. Size the impeller to give a flow velocity in the suction pipe of 1 to 2.5 meters per second (3 to 8 feet per second) or increase the size of the suction pipe if a large pump is required.
Where negative pressures (vacuum) cannot be avoided they should be prevented from exceeding about two-third the difference between atmospheric pressure and the vapor pressure.
Suction piping design is more important than the discharge piping design. Use long radius elbows, use full-bore valves, make smooth changes to cross-sections, keep the pump as far below the source as possible, keep branches at least 10 pipe diameters up stream of the suction flange and have easy access to strainer screens for operators to clean.
Provided the pump produces more pressure than necessary, machining the impeller diameter down can reduce the velocity. Adding inducers to the impeller can reduce pipe friction. An inducer is a large pitch screw that fits down the suction pipe and draws the liquid into the pump. They can reduce pressure loss by up to 50% of that which would have occurred.
1.14 Welding Metals
Welding metal together is done by locally melting the edge between two parts and letting the molten puddle formed by the parent metals and the filler metal solidify. As the metals cool the atoms arrange themselves into microscopic crystal grains jammed together, much like jigsaw puzzle pieces. In-between neighboring grains are microstructures of other chemicals in the metals. The chemical make-up, the size, and arrangement of the grains and grain boundary matrix determine the strength and the properties of the weld. Well mixed, fine, evenly distributed grains in a thin grain boundary matrix is best while big, uneven grains and a wide grain boundary matrix is worst.
As the weld cools down from the edge toward the center there is a difference in grain properties and grain sizes across the weld, with the most poorly formed grains being at the edge where heat loss was fastest. Molten metal cooled-off fast freezes the microstructure in the wrong form and is hard, brittle and full of micro-cracks. Molten metal cooled down at a slow, controlled rate consists of well-shaped crystals with alloy elements well distributed throughout the microstructure and few micro-cracks.
The temperature gradient in the parent metal, from the melt temperature at the edge of the weld to the metal temperature well away from the weld, takes its atomic structure through a heating and a cooling down period. Where the parent metal gets hot enough chemical reactions occur and the microstructure changes. The grains and the grain boundary matrix alter. Not only does a weld change the metallurgical structure in the melted metal, it also changes the metallurgical structure in the metal around it. This region near the weld where the microstructure changes is called the Heat Affected Zone (HAZ).
If poor metallurgical changes occur during the welding then its quality is low and the weld has a greater chance of failing. Once a part is welded the rate of cooling in the weld and HAZ must be slowed to acceptable limits. Tables and charts specifying cooling rates are available from welding material suppliers. The usual practice is to wrap the area around the weld in insulation. If the section is thick and the cooling is done in a draft free area it may be unnecessary to use insulation.
Weld Preheating
Weld preheating is the purposeful application of heat to warm metal to a desired temperature prior to welding. Preheating slows down the cooling rate of the weld and gives the metal more time to form a good microstructure, release internal stresses and dissipate hydrogen from the weld.
The cooling rate affects the weld's final properties such as hardness and ductility. Preheating prior to welding will:
· Reduce the chance of catastrophic cracking in the weld from micro-cracks and trapped hydrogen gas.
· Reduce the hardness and brittleness of the weld due to rapid cooling.
· Reduce the amount of distortion caused by the weld introducing stress into the part.
· Reduce the amount of shrinkage stress from the differential temperatures present between the weld and parent metal.
The metal in all three dimensions around the weld puddle is raised to the predetermined temperature (up to 300oC (570oF) for steel) before welding and then allowed to cool-off slowly once welded. Without adequate preheat, the cooling would be rapid and intolerably high hardness and brittleness would occur in the weld and the heat affected zone neighboring the weld.
Common materials that require preheat are steels, cast irons, copper and its alloys and aluminum. Often the heat from welding is sufficient to preheat the metal. However preheating the weld is required when the metal:
· is below 20oC
· conducts heat away very fast (such as aluminum, copper and both thick and thin steel sections)
· requires slow cooling to form the correct microstructure after melting (like cast irons and thin steel sections)
· will be highly stressed when in use (pressure vessels, lifting equipment, etc)
If situations arise where preheat cannot be applied there are several options which can be adopted.
· Use low hydrogen electrodes to reduce the risk of cracking.
· Peen the weld as it cools off with a blunt pointed hammer. The hammer blows from peening the weld vibrate the microstructure and tend to break it up into finer crystals.
· Use multiple weld passes to seal the join. Each subsequent pass tends to heat treat the preceding welds and provides a cover to reduce the rate of heat loss.
1.15 Welding Plastics
Plastics have some wonderful engineering properties and priciples that can be applied to great benefit.
· they handle a vast range of chemicals;
· they don't rust;
· some of them are very slippery and little sticks to them;
· they are extremely cheap compared to the exotic alloys required to match some of the properties;
· they don't transmit electricity or heat easily
· some are tough and will deform instead of breaking under impact
· they are so easy to fabricate that people can be trained in a week to join plastics well.
Their major drawbacks when compared to metals are:
· most soften at comparatively low temperatures;
· they cannot take high tension continuously;
· some can expand greatly when heated;
· they breakdown in sunlight unless protected.
The two families of plastic
Plastics are broadly grouped into two distinct families 'thermoset and thermoplastic. Thermosets can only be molded once. After their first melt they set permanently. A thermoplastic can be melted a number of times and the shape changed. Welded plastics are thermoplastics.
The difference between the two families of plastic results from how the macromolecules bond together at the atomic level. Thermoset molecules are triggered by heat to chemically react and join. Thermoplastic molecules are attracted to each other but do not chemically bond. Figure 1 shows the difference in the type and numbers of bonds between thermoset and thermoplastic materials.
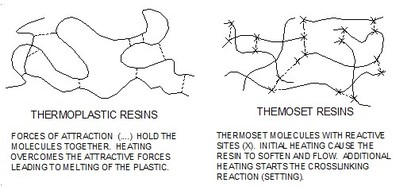
Heat joined plastics
The common plastics, which are joined together using heat, are PE (polyethylene), PP (polypropylene) and PVC (Polyvinylchloride). At the exotic end PVDF (polyvinylidene fluoride) and PTFE (polytetrafloroethylene) can also be welded.
Joining plastic together
Heating the contact surfaces above their melting point and then pushing them together firmly till they set joins thermoplastics. When the molten faces come together the macromolecules intertwine and bond together on cooling. The three critical factors for a good join are - achieving the right melt temperature; sufficient pressure when pushing the faces together; the length of time the join is allowed to cool before releasing the pressure.
METHODS TO JOIN PLASTICS USING HEAT
Butt-welding is used to join pipes. The pipe ends are held in a special clamping rig, then cut and faced square with a cutting tool. A hot plate set to the melt temperature is inserted between the two ends and the pipes pushed onto the hot plate. Once enough time has passed to melt the pipe ends the hot plate is removed and the ends pushed together under pressure. After a time ranging from a few seconds to a few minutes, depending on the thickness of the pipe, the pressure is released. The pipe is then left to cool down. This may take from a few minutes for small-bore pipe and up to an hour and a half for large pipes with 50 mm wall thickness.
Socket welding is done by using heated tools to melt the outside of the first few centimeters of a pipe end and the first few centimeters on the inside of the socket fitting. The pipe is pushed inside the socket and held in place till it cools.
Electro-socket welding is the same as socket welding except an electrical wire is installed in the fitting when it is made. The pipe is pushed into the socket and the wire connected to a power source. The wire heats up and melts the plastic surfaces. When the power is removed the plastic cools down. Beware that the metal heating wire can come into contact with the process chemical in the pipe. If the chemical is incompatible with the metal it will corrode the filament and leak out along the wire track.
Extrusion welding is used to weld plastic sheets together. Plastic wire from 2 mm to 6 mm in diameter, depending on the size of the extruder, is fed through a heated barrel where it melts. The tip of the extruder heats the plastic sheet and melts the surface. The molten wire in the extruder is forced onto the melted surfaces and joins the edges of the sheets together. The extruder is gradually fed along the joint melting the surfaces and laying the filler material as it goes.
Extrusion welded joints are de-rated to 80% of the parent material's strength.
Hot air gun welding is used for light duty fabrication and tacking large fabricated items together before finally extrusion welding them. With this method a hand-held plastic wire is pushed into the joint made by the corner edges of the two parts. The hot air gun is used to melt the corner edges of the plastic and the hand-held plastic wire. The operator watches for the melt to develop and forces the wire into the weld. The hot air gun is kept ahead of the moving melt and the plastic wire continuously rolled forward into the puddle. Cooling is rapid and by the time the operator's hand passes a point the plastic has joined together.
Good plastic welding practice.
The secret to a high quality plastic weld is cleanliness. Contamination must be avoided. For example water will cause voids and bubbles in a weld. The contacting faces or edges must be clean. Before heating a butt or socket weld acetone is used to wipe the end of the pipe clean. Recognized procedures need to be properly followed and operators trained and tested to the procedures. Most plastic welding procedures are based on German standards. The quality of butt welds can be checked by tensile tests. A sample of a butt-welded pipe is held at each end in a machine and stretched. The force is measured and the weld must stay together up to the required load.
A spark test can be used to check extrusion welds. A voltage created by an electrically charged plate on one side of the sheet and an oppositely charged hand held wire brush on the other side will cause a spark to jump if a hole is present.
Good practice is to always test welds with water under full operating pressure. In the case of piping this will confirm its integrity and with tanks it will locate leaks.
1.16 Heat Transfer
Heat is energy and its nature is to flow from a state of high excitement to one of low excitement. Heat is transferred from a hot place to a cold place by convection, conduction or radiation. Lets explorer the applied engineering principles behind heat transfer.
Radiation is the transfer of heat energy by the movement of electro-magnetic radiation through open space. All matter in the universe radiates energy unless its temperature is at absolute zero (-273 oC). Emitted radiation will be absorbed into, reflected from or transmitted through a body in the path of the radiation.
Conduction is the transfer of heat from one part of a substance to another, or to second substance in contact with it. Heat energy moves through a body by exciting neighboring atoms. A temperature gradient (slope) is created from the hotter to the cooler sections.
Convection is the transfer of heat within a fluid (liquid or gas) by the mixing of one portion of fluid with another. The mixing can be by changes in density (e.g. hot air rising over an open fire) and is called natural convection or by mechanical means (e.g. a room fan heater) and is then known as forced convection.
A hot surface will transfer heat to a fluid (liquid or gas). In order to boil water for a cup of tea or coffee, the energy from a kettle's heating element must be transferred to the water. The water nearest the element touches the surface and gets hot by contact (conduction). Natural convection causes the hot water to rise and new, cooler water touches the element and gets heated.
Convection can also be by mechanical means (known as forced convection) such as pumping through heat exchangers, using static mixers inside tubes to make the flow turbulent and putting agitators in steam jacketed tanks. Forced convection is used because the fluid is forced into contact with the hot surface many times during its passage around the heat transfer equipment. This increases the heat input and warms the fluid faster.
Heat Loss Minimization.
Where heat loss is to be reduced it is necessary to:
§ reduce heat transfer coefficients,
§ reduce thermal conductivity (or increase thermal resistance),
§ use low emissive surfaces,
§ increase the thickness of the walls,
§ keep surface areas small,
§ block the direct radiation, conduction or convective paths
between the two substances,
§ minimize the temperatures of surfaces,
§ minimize temperature difference with the surroundings.
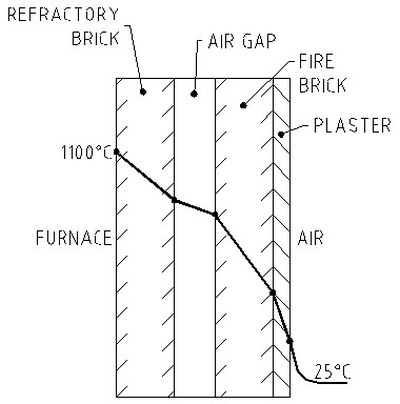
Radiated heat whose escape is to be minimized (like the flame in a boiler) is usually 'boxed-in'with thick walls made of low conducting materials like refractory bricks. These are then insulated on the outer side with low conducting, low emissive materials like fiberglass blankets. This sandwich construction offers high resistance to heat transfers (i.e. low conductivity). For example, the flame temperatures in a boiler maybe 1000 oC (1832 oF) but the outside wall can be touched by hand.
An example of the use of multiple materials to create a thermal resistance is shown in Figure No. 1. It is a cross-section through a furnace wall showing the temperature of the various surfaces that make-up the wall (the temperature gradient). Note that the air gap stops conduction as the main heat transfer mode and makes radiation the dominant mode. If fiberglass batts could be used to fill the air gap, or aluminum metal sheeting hung between the walls, radiation would have greatly reduced and the outside wall temperature would be even lower.
In a steam jacketed tank the inside wall is made as thin as possible to reduce the thermal resistance. The outside wall of the jacket is insulated to prevent heat loss to the cold surroundings. If it were not insulated the outer wall of the steam jacket would radiate heat into the cold surroundings. An un-insulated, jacketed tank would waste a lot of steam to heat the contents. Much heat would be lost as radiation from the tank's outer wall and as convection of the air touching the outside wall. Insulation saves a great deal of otherwise wasted energy.
Heat Loss Maximization
In situations where maximum transfer of heat is required it becomes necessary to find ways to:
§ make the fluid contact the hot surfaces as much as possible by using turbulence,
§ keep the temperature difference between the surface and the fluid as great as possible,
§ keep the thermal resistance of materials separating cold fluids from hot fluids the as low as possible,
§ get the heat transfer coefficient of the fluids up,
§ remove any surface scaling,
§ use materials of high thermal conductivity (i.e. low thermal resistance).
For example, boiling a pot of water over an open fire in the forest takes longer than boiling the same pot over a fireplace hearth in a house and both take longer that boiling the same amount of water in an electric kettle.
Out in the open air, the pot gets only a small amount of the fire's heat. Most is radiated in all directions. Some radiation hits the pot and some convective heat from the hot rising air touch the pot. In the hearth the pot gets radiation off the hot surrounding bricks and the fire and also convection heat from the hot rising air. In the kettle the water gets heat from direct contact with the heating elements. All the heat in the kettle's heating elements is injected into the water and little is lost to the surroundings.
When trying to heat-up a substance it is necessary to insure that the maximum heat possible is put into it and not lost to the surroundings.
1.17 Bearing isolation and protection
Bearings are contained within a housing from which a shaft extends. The shaft entry into the housing offers opportunity for dust (and moisture) to enter the bearing. The shaft seal performs sealing of the gap between the housing and shaft. Choice of the appropriate shaft seal and seal configurations to protect against dust ingress is critical.
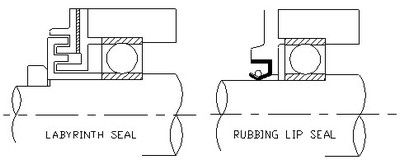
Bearing housing seals for dusty environments may be either a labyrinth type or a rubbing seal type. The labyrinth type requires a straight shaft running true. Rubbing seals are the more common and allow for some flexing of the shaft. The sketches below are conceptual examples of each type of seal. When setting a lip seal into place to prevent dust ingress insure the sealing lip faces outward.
In situations of high dust contamination there may be a need to redesign the shaft seal arrangement for better dust protection than provided in standard housings. Some ideas which can reduce dust ingress into bearing housings are to :
i. Provide two or more seals in parallel. Bearing housings can usually be purchased with combination seals as standard.
ii.Retain the housing shaft seals but change from a greased bearing in the housing to one which is sealed and greased for life. If contamination were to get past the shaft seals, the bearing's internal seals would protect it.
iii.Stand the bearing off the equipment to create a gap between the end of the equipment and the bearing housing while sealing the shaft at the equipment.
iv. Put in a felt seal wipe between the housing and the wall of the equipment to rub the shaft clean. Install of a mechanical seal in very harsh environments.
v.Install a grease barrier chamber sandwiched between two seals. This barrier is separate to the bearing housing and acts as the primary seal for the bearing. Grease pumped into the chamber will flush out past the seals.
vi. Replace the grease barrier chamber instead with an air pressurized chamber.
vii.Shield the bearing housing from dust with use of a specially fabricated rubber shroud encapsulating the housing and wiping the shaft or fit a rubber screen with a hole wiping the shaft over the opening emitting the dust.
viii. Flush the bearing with grease by pumping excess grease into the housing and allowing the grease to be forced past the shaft seals or through a purposely drilled 15 mm hole in the housing. The hole must be on the opposite side of the bearing to the grease nipple, at the bottom of the bearing housing when in service and between the bearing and seal.
ix. Mechanical seals can be fitted to the shaft with the stationary seal sitting toward the machine and the rotating seal mounted back along the shaft. Combinations of other seals and wipers can also be used in conjunction with the mechanical seal. Mount the auxiliary seals so they see the dust/water first and keep the mechanical seal as the last line of protection.
Assembly
The process of assembling a bearing into the housing must be spotlessly clean. If contamination occurs at the time the housing is assembled no amount of external protection will stop the bearing from premature failure. When assembling bearings into housings make sure that:
i. Your hands have been washed.
ii. The work bench is clear and wiped down clean.
iii. No one creates dust or grinds nearby during assembly.
iv. Fresh, clean grease is used to pack the housing.
v. The components are clean and all old grease has been thoroughly removed.
Breathers
When protecting bearings from dust you want to always consider another important area. A breather is used to let hot air out of a confined space and then to let the air back in when it cools down. Enclosed bearings get hot when operating and cool down to ambient temperature when not in use. The air drawn back into the space needs to be clean of dust and moisture. A breather on a bearing housing or bearing housing enclosure allows ingress of moisture and dust into the bearings causing premature life failure.
Often a breather is insufficient and should be replaced with a low micron air filter that removes dust particles two micron and greater in size. Protect the breather or filter from water spray and damp conditions (ban hosing down if possible) with a shroud or by using an extension tube going into a clean, safe environment. Make sure the breather tube cannot be crushed closed by accident.
1.18 Electric Motors
Induction motor design
An electric motor consists of an iron rotor wheel mounted on a shaft, supported by bearings at each end, spinning within a multi-coil cage of wire called a stator. Copper or aluminum bars are imbedded in the outside surface of the rotor and connected together to form a circuit. The wire windings in the stator are arranged to form an electromagnet. Figure 1 shows a simplified motor design. The electric currents flowing through the outside stator coils create a magnetic field through the rotor while inducing an electric current in the rotor bars.
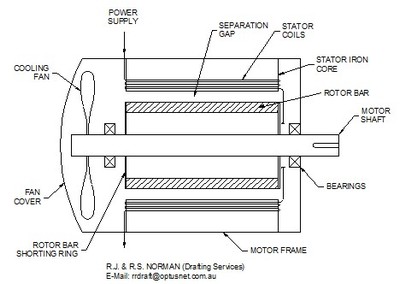
When alternating current (AC) flows through the stator coil, reciprocating north and south magnetic poles are created at the ends of each coil. At the same time, like a transformer, the electric fields in the stator coils also create an electric current in the rotor. When an electric current is cut by a moving magnetic field a reaction force occurs in the current carrying conductor. The bars in the rotor, now induced with current, react in response to the magnetic field and force the rotor to turn. The alternating magnetic field is then created in the neighboring coil and the rotor continues to turn.
For motion to be induced on the rotor the electric carrying conductor must cut the magnetic field. This means the rotor must move slower than the cycling magnetic field. It is only by cutting through the lines of magnetic force that torque is generated on the rotor. An electric motor will always run at a slightly slower speed than the cycling magnetic field.
The motor speed depends on the number of separate magnetic fields created by the coils in the stator. A two-pole motor has one coil and one magnetic field arranged around the stator, a four-pole motor has two coils arranged around the stator with each winding placed between the other in sequence. A six-pole motor has three coils with the windings spaced in sequence around the stator, and so on.
Operation of an induction electric motor.
An induction electric motor works when AC power is turned on. Each phase of the alternating current goes into motor windings positioned around the outer stator. The position of the stator windings and the rise and fall of the alternating current produce sequentially rotating electric and magnetic fields inside the stator. The rotor is free to turn inside the stator. Within it the rotor has metal bars. The stator electric field induces an electric current in the conducting metal bars (like a transformer). When a magnetic field cuts through a conductor carrying an electric current a force is developed in the conductor at right angles to the magnetic field. When the circulating stator field energizes the metal bars in the rotor they are forced to move and the rotor turns (See article 262 Electric motor problems and 334 Electric motor current protection saves your plant).
It is important to understand that there must be relative movement between the conductor and the magnetic field. The force is only developed if the magnetic field cuts the conductor. In a motor the rotor runs slower than the rotating stator magnetic field. This means the magnetic field cuts the rotor conductors. The difference between the speed of the magnetic field and the speed of the rotor is called 'slip'.
The greater the difference between the stator magnetic field speed and the rotor speed (the 'slip') the more often the magnetic field cuts through the conductor and the greater the force generated on the conductor.
Electric motor torque and power characteristics.
Because an electric motor rotates it can only deliver a torque (a turning force) through its shaft. It also means that an electric motor will experience all equipment loads on it as a torque. The motor shaft will turn and the attached equipment will move only as long as the motor can generate the required torque. Applied engineering principle - As the rotor speed drops because of an increased load the rotor slips more, the torque generated rises and the current draw in the stator increases.
The torque generated on the rotor and attached shaft results from the interplay of several electrical, magnetic and physical variables that alter with the speed of the rotor. The motor's behavior is a response to the load imposed on it by the equipment connected to it. The equipment's behavior is itself a response to the duty and service it has to perform. Where the equipment duty and service is adjustable by the operator, or fluctuates as the process changes, the motor will react to those changes. When the changes are so excessive that they are beyond the motor's ability to handle them the motor stops and the equipment or process stops with it.
When choosing an electric motor it is necessary to consider if the behavior of the load attached to the motor is suited to the load characteristics of the electric motor. A rotor begins from rest and must come up to full speed while dragging its load around with it. The electrical currents that occur within a rotor going through start-up and operation vary greatly and influence the motor load carrying capacity.
Problems with electric motors
Below are a number of problems that are often encountered when using electric motors.
§ Water ingress into the motor will go between the stator coils or into the terminal box and short circuit and burn out the motor. Water must never be allowed to get into a motor. If motors are to be used in wet areas that must be of the correct ingress protection (IP) rating.
§ Overheating can occur from under-sizing the motor, insufficient cooling at low speed when using variable speed drives (VSD), changes to the load on the motor such as jammed equipment and hot ambient conditions. Temperature detection (thermistor) and automatic shut down devices can be installed. Attaching a separate booster fan to aid the motor fan solves the overheating problem when a VSD or VFD is used to control the motor speed.
§ Bearing failure on motors can be an indication of the incorrect bearings for the application. A motor mounted vertically needs different bearings to a motor mounted horizontally. A motor driving a large or multi-belt drive will require bearings that handle big radial loads. A motor bolted to a distorted base plate will twist (See Soft Foot). Check bearing types with the manufacturer.
§ Motors in-store or not in operation for long periods of time get false brinelled bearings where the bottom bearings etch into the shaft. Turn the motor shaft a quarter turn monthly. Bearings in motors in-store exposed to low vibrations through the ground can brinell. Sit motors on a sheet of 3-mm rubber to insulate them from ground borne vibrations.
§ Burnt windings imply a short circuit either within the motor or within the power supply circuit for the motor. Over-current protection can be installed as part of the power supply circuitry.
§ Dust ingress into the stator coils or the terminal housing leads to short-circuiting. If the motor is to be in a dusty environment keep the immediate area around the motor clean or use dust ingress protection (DIP) methods.
§ Hazardous area motors must comply with the type of hazard in the area. Motors in flammable vapors like gasoline, in explosive vapor atmospheres and in explosive dust environments such as grain dust all need to be rated and protected for the specific location. There are various methods of hazardous area motor protection but they are not transferable across hazard types. For example a motor protected against an explosive dust is not suitable to use in a flammable environment.
§ Temperature ratings of housings vary. The motor housings get hot under operation and there are six different temperature ranges available for motors depending on the environment it must work in.
§ Running in reverse is a common problem. Changing over any two terminal leads changes the motor direction. Always test run a motor to check direction after it is wired-up. Separate the shaft coupling to protect the driven equipment from damage if necessary. Automatic current reversing relays are available to provide the correct motor direction.
§ Shaft misalignment will destroy bearings well before their full working life. The motor shaft must be directly in-line with the shaft it is driving. This can only be achieved by using precision alignment techniques such as laser or dual dial indicators. The motor shaft must turn along its full length to within 0.05 mm (0.002') of the true center of the driven shaft. This minimizes the vibration, forces and loads that planetary rotation of one shaft in respect to the other would create.
§ Soft foot occurs when the motor feet are bolted down out of level. If all feet are not in the same plane when pulled down on the base plate the motor housing twists and the bearings are distorted. Put a straight edge across the base plate and measure gaps with feeler gauges. Place the motor on a flat, machined bed and check the gap under each foot. Put 316 stainless steel shims under the high feet to level them when bolted down.
§ Wrong motor mounting or housing type leads to frustration. Motors are either foot mounted or flange mounted. They come in various frame sizes and designs and the correct choice and delivery is needed.
Tap this resource if you would like to see the full interactive Electric Motor Troubleshooting Flowchart at our subsidiary Koldwater.
Proper handling of motor load changes.
In all cases loads on electric motors should be increased slowly and must be less than the abilities of the motor to generate the required torque. If pump or fan motors trip out check that the valves or dampers are positioned to keep flows within the motor's load capacity. If motors on equipment trip or burnout, then somewhere there must be something causing a big load. Find the load and remove it before again starting the motor.
One way to assist people in monitoring loads on electric motors is to install current monitoring read-outs and set working limits for the motor that are not to be exceeded. People can then monitor the affect on the motor of process flow, pressure or load changes that they make.
1.19 Process Control and Monitoring
A control loop senses, monitors and adjusts a process to maintain it within preset requirements. The loop consists of instruments, computers and computer-controlled equipment that interact to make continuous fine adjustments. Operators are required to run their plants from control panels. They view the control panel dials, lights and readouts and from them they interpret the plant and equipment operating condition. It is critical that every indicator on the panel be a true reflection of the equipment's state out in the plant.
If a control panel indicator means something is on or off then it must reflect the true physical operating condition of the equipment. Anything different confuses people, leads to errors and wastes money to fix the damage that must eventually occur.
The sole purpose of using process control in, say, the making of beer, is to detect what is happening during manufacture and to decide if something needs to be changed to insure the beer meets the necessary specification. The key words are 'to detect', 'to decide', 'to change'and 'to meet specification'. A control loop can do all these things automatically.
Control loop components
A control loop is made up of the components shown in Figure No 1. It consists of '
· a sensor to detect the process,
· a transmitter to make the sensor signal into an electrical signal,
· a controller that tests that the signal meets a pre-set value and if not makes the adjustments required,
· a final controlling element(s) that physically move and alter its position (e.g. a control valve),
· a converter that changes an electrical signal into a proportional force or a force into an electrical signal (a transducer),
· the medium (like steam, chilled water, etc) that provides the energy change to the process to produce the correction.
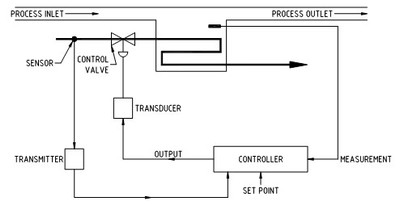
Open and closed loops
Very simple control loops that detect an incoming process variable and display the value to an operator are called 'open loop'control systems. In this case it is up to the operator to make any changes to the process. As soon as a final control element is added to allow incoming process variable adjustment the complexity and cost of the loop rises sharply. The final control element requires a decision-making device (the controller) to signal it to move. Such an arrangement is called a 'closed loop'control system because the sensor's signal controls the reaction of the loop from the initially detected process change right through to the finally detected adjustment. The entire change starts and ends (closes) with the sensor detecting the proper required conditions.
Feed back 'feed forward systems
The requirements for the speed and accuracy of the control loop response determine whether 'feed back'or 'feed forward'control is used. Feed back control measures the final product and checks it is produced to within the specification tolerance. If it is not it within tolerance the final control element is adjusted to make corrections.
Feed forward control measures the incoming variables before they go through the process, making sure they are within preset tolerance before combining together to make the product. Feed forward control is the more complex and expensive method to use.
One can see that it is best to use the least complex and inexpensive control loop philosophy possible. Complex loops require expensive components; great amounts of effort programming and tuning the controller; are easily 'confused'by abnormal process occurrences; require unending maintenance to retain their sensitivity; demand high levels of emergency spare parts and the presence of highly skilled operators and maintainers.
The right control loop choice can only be made when the product specification tolerance is well understood. If low cost control is required the product specification must have a wide range of customer acceptability. Tight tolerances add great cost.
Care should be paid as to where feedback sensors are installed so that all possible modes of failure are detected. Mounting a motion sensor on the drive end of a screw conveyor will not detect a screw shaft broken half way along it. But mounting the sensor at the discharge end will detect such a failure. Alternatively, torque monitoring of the electric motor could be installed and a signal sent to the operators when the torque varied from an acceptable range.
Process Logic Controller (PLC)
A process logic controller is a computer designed for monitoring and controlling equipment. The PLC accepts signals (inputs) from sensors located throughout a piece of equipment or process plant. It then follows the instructions (program) within its memory and sends out commands (output) to operate equipment.
Inside the PLC
Modern PLC's are a modular construction of electronic components on circuit boards that individually plug into place. There are four basic parts 'the Input Module (input card), the Processor Module (CPU), the Output Module (output card) and the Power Supply. The heart of a PLC consists of the processor module. It receives signals from the input module and then follows the program to generate signals that are sent out by the output module. Figure 1 is a simple overview of a PLC's interactive functions.
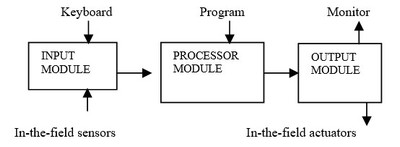
Humans interact with a PLC by use of monitors and keyboards. Special programming panels with a keyboard and screen connect to the PLC and allow the control program to be put into its memory. When the PLC is in use we view its instructions and the resulting actions by a screen monitor. This can be mounted on the control room desk or near the equipment being controlled. A keyboard or mouse is used to give a command signal to the PLC when it is operating.
Connecting the plc to the world outside
The PLC is a computer and operates with digital instructions in binary code of '0'and '1'. However field sensors produce an electrical signal in proportion to the effect they are monitoring (analogue signal), while the field actuators operate either by electrical or pneumatic (air pressure) means. The PLC must receive binary information to use the control program and perform the required instructions. The analogue electrical signals must be converted into digital signals and digital signals into analogue electrical signals. This is the function of the input and output cards (the I/O).
The input module converts a sensor's electrical signal into its digital binary equivalent signal. While the output module does the opposite and generates an analogue electrical signal that go to the field actuators, from the digital instruction provided by the PLC. Interconnecting all the sensors, PLC and actuators is the communication cabling.
The data communications cabling carries low voltage electrical signals. These signals are the means by which the PLC interacts with the equipment it controls. The data cable does not provide the electrical power to move actuators. A separate power supply is provided to the actuator for its operation. Instruction signals from the PLC need to activate an individual item of equipment. At present there are three commonly used methods to transmit signals between the PLC and the field sensors and actuators to activate the equipment.
Issues with using PLC's
The PLC is inseparable to the safe operation of the plant and equipment. Without the PLC the plant cannot be safely run. It is possible to configure the PLC so it can be removed from its controlling function and the whole plant operated manually. This may be a useful feature to jog and test individual equipment after repair but it is a dangerous practice to run plant in manual when the PLC fail-safes and protection are inactive.
Very few control software programs work properly on their first installation into the PLC. Most times they require some degree of modification under field conditions. Unless the field signals are properly interpreted by the PLC and its instructions are then properly sent and properly received by the field actuator, a fault will occur. Often it is necessary to install sensors on the field equipment to actually confirm the instruction has been performed.
Ad-hoc (spur of the moment) control program change is another dangerous practice to adopt. Often when a plant is operating it becomes clear that if it worked in a slightly different way it would perform better. So the required changes are quickly and suddenly made to the program and the plant put back in service. Many times the change affects something else that was not recognized as being affected. Suddenly the plant's operation is in jeopardy. Ad-hoc program changes lead to trouble, each change must be thoroughly investigated while using engineering principles applicable, and tested by plant knowledgeable persons before it is made permanent.
Keep a copy of the new and old programs. Every time a change is made to a program three things must be done. First the reason for the change and the results of the investigation must be permanently documented for auditing and traceability. Secondly a copy of the new program with the changes must be made and kept in a safe place away from risk of damage. Thirdly a complete copy of the program prior the change must be kept so the plant can be put back into its previous configuration should the new program change cause problems.
Standardize on the PLC to be used throughout the plant. A PLC requires support from people to do repairs on its parts and to modify its program. It takes much effort to become proficient (to master) in supporting a PLC. If there are more than one type of PLC on site then two sets of spares are required and two sets of training and two programming panels are needed and two lots of program copies must be kept. If someone then buys in a third type of PLC for a new plant the problem of support becomes monstrous. The answer is to standardize on one PLC supplier.
If you would like to reference this guide offline, and/or print copies for your maintenance, operators, or students in your facility, you can purchase a PDF version of this guide below.
On to chapter 2, Equipment Maintenance Best Practice ...

